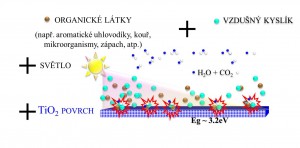
Brief review of photocatalysis principle
Photocatalysis on TiO2 surface represents a promising alternative technology for degradation of organic pollutants and inactivation of microorganisms.
Photocatalysis is a purely physical semiconductor effect depending on the band gap (difference between valence and conduction band) of the particular semiconductor. In the case of TiO2 based photocatalysts it is a substance unrelated effect that functions independently on chemical composition.
A photocatalyst is a material which absorbs light to bring it to higher energy level and provides such energy to a reacting substance to make a chemical reaction occur. Photocatalyst function is based on the photogeneration of separated electrons and positive holes in semiconductor particles. These charge carriers either recombine inside the particle or move to its surface, where they can interact with adsorbed molecules.
Positive holes typically oxidize organic compounds, inducing their oxidative degradation, while electrons mainly reduce molecular oxygen (if present) to superoxide radical anions. The governing phenomenon of photocatalytic degradation is direct charge transfer of photogenerated holes.
Scientific Board of the Czech Association of Applied Photocatalysis
Prof. Josef Krysa (University of Chemistry and Technology, Prague)
Dr. Jiri Rathousky (J. Heyrovsky Institute of Physical Chemistry)
Prof. Michal Vesely (University of Technology, Brno)
Dr. Jan Subrt (Institute of Inorganic Chemistry AS CR)
Prof. Ladislav Kavan (J. Heyrovsky Institute of Physical Chemistry)
References:
[1] Surface Science, Volume 13, Issue 1, January 1969, Pages 265-278
H.Gerischer, https://doi.org/10.1016/0039-6028(69)90257-X
[2] Energy Reports, Volume 6, Supplement 4, February 2020, Pages 2-12
Ronald Vargas, David Carvajal, Lorean Madriza, Benjamín R.Scharifkerab
Chemical kinetics in solar to chemical energy conversion: The photoelectrochemical oxygen transfer reaction, https://doi.org/10.1016/j.egyr.2019.10.004
[3] Applied Catalysis B: Environmental 64 (2006) 290–301
Josef Krysa, Georg Waldner, Hana Mestankova, Jaromir Jirkovsky, Gottfried Grabner
Photocatalytic degradation of model organic pollutants on an immobilized particulate TiO2 layer
Roles of adsorption processes and mechanistic complexity, https://doi.org/10.1016/j.apcatb.2005.11.007
[4] Catalysis Today 182 (2012) 39– 45
I. Ross Macdonald, Shona Rhydderch, Emily Holt, Neil Grant, John M.D. Storey, Russell F. Howe
EPR studies of electron and hole trapping in titania photocatalysts, https://doi.org/10.1016/j.cattod.2011.08.039
[5] Solar 2022, 2(2), 305-320
Thaleia Ioannidou , Maria Anagnostopoulou and Konstantinos C. Christoforidis
Two-Dimensional Photocatalysts for Energy and Environmental Applications, https://doi.org/10.3390/solar2020017
[6] Science of The Total Environment
Alexandre Albinet, Claudio Minero, Davide Vione
Volume 408, Issue 16, 15 July 2010, Pages 3367-3373, https://doi.org/10.1016/j.scitotenv.2010.04.011
[7] iScience, VOLUME 25, ISSUE 4, 104107, APRIL 15, 2022
Kristijan Lorber and Petar Djinovic
Accelerating photo-thermal CO2 reduction to CO, CH4 or methanol over metal/oxide semiconductor catalysts, https://doi.org/10.1016/j.isci.2022.104107
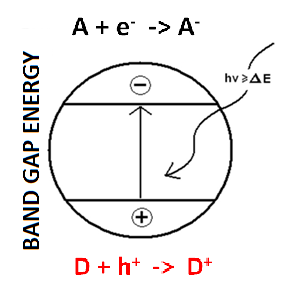
Citation Excerpt :
The foundations of semiconductor electrochemistry were laid by the works of Brattain and Garrett (1955) and Garrett and Brattain (1955), who introduced the use of Nernst-type potential expression involving the activity of electrons and holes. Additionally, the pioneering contributions of Gerischer (1969a, b, 1966) allowed linking the anodic or cathodic current at semiconductor electrodes with the availability of excess of holes in the valence band or excess of electrons in the conduction band, respectively. Gerischer introduced the concepts of minority charge carriers and density of states with the concomitant development of an extensive theory related to the physical and chemical processes that concur at the semiconductor–electrolyte interface (Gerischer, 1990, 1991, 1993, 1995; Memming, 2015; Sato, 1998; Morrison, 1980).

